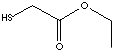
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
THIOGLYCOLATE /
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
'Thio' is a chemical prefix indicates the replacement of an oxygen in an acid radical by sulfur with a negative valence of 2; meaning 'sulfur' derived from the Greek theion. In substitutive nomenclature their names are formed by adding '-thiol' as a suffix to the name of the parent compound. When -SH is not the principal group, the prefix 'mercapto-' is placed before the name of the parent compound to denote an unsubstituted -SH group. Thioglycolic acid, a simple sulfur group- chained carboxylic acid, is a clear liquid; melts at -16. c, boils at 96 C; soluble in water. It is an useful chemical intermediate in the chemical reactions such as addition, elimination and cyclization. Sulfur group will react with bases, acids, ketones and organic halogen compounds, whereas the carboxylic group will preferentially react In the presence of alcohols or amines. The applications of thioglycolic acid and its derivatives are wide in the fields of;
- Main materials for the synthesis of PVC stabilizers.
- Down-hole acidizing
- Corrosion inhibition in the oil field industry.
- Manufacturing of pharmaceuticals, agrochemicals and dyes
- Hair care products (waving, hair removal and hair straightening)
- Shrink-resistant treatment of wool
- Fabric dying
- Leather processing.
Thioglycolic acid, a simple sulfur group- chained carboxylic acid, is a clear liquid; melts at -16. c, boils at 96 C; soluble in water. It is an useful chemical intermediate in the chemical reactions such as addition, elimination and cyclization. Sulfur group will react with bases, acids, ketones and organic halogen compounds, whereas the carboxylic group will preferentially react In the presence of alcohols or amines. The applications of thioglycolic acid and its derivatives are wide in the fields of PVC stabilizers, down-hole acidizing, corrosion inhibition in the oil field industry, manufacturing of pharmaceuticals, agrochemicals and dyes, shrink-resistant treatment of wool, fabric dying, leather processing.
Thioglycolates are used in cosmetic hair-care products especially permanent waving products as they weaken the keratin structure by the opening of the cystine disulfide linkages. In addition to hair-waving application, they are used as depilating agents to remove unwanted body hair. The break-down of disulfide bonds in the cortex by thioglycolates either rearranges (permanent waving) or entirely destroys (depilating) hair structure. As an antioxidant application in cosmetics, thioglycolates protect the product itself not the skin against oxidative reactions promoted by ultraviolet radiation or oxygen.
|
|
APPEARANCE
Clear liquid
ASSAY
99.0% max
PRICE